Figure 1. Map of Ethiopia showing the catchment areas of the hospitals included in this study
ORIGINAL ARTICLE
Bernt Lindtjørna, Thor Henrik Henriksenb
a MD, PhD, Professor, Centre for International Health, University of Bergen, Norway and Hawassa University, Southern Ethiopia
b PhD, Department of Microbiology, Vestfold Hospital Trust, Tönsberg, Norway
Epidemics are often seen at mission health institutions. Such Christian institutions seek to practice holistic medicine, and the core priorities include dedicated clinical care combined with community responsibility. This paper describes some unusual, and some more common, epidemics that occurred at three mission hospitals in Southern Ethiopia during the last 60 to 70 years. The hospitals covered vast areas and large populations, mostly from poor subsistence farming communities. With great topographical and climatic variations, the catchment areas include multiple climate zones that cause substantial variations in ecology and vegetation, and thus, also in disease patterns. Our review is based on personal notes, hospital records, and previous scientific publications.
We observed epidemics such as cholera and other diarrheal diseases, relapsing fever, meningitis, gonococcal conjunctivitis, the emerging of HIV and Helicobacter infections, and parasitic infections, such as malaria and visceral leishmaniasis. Hospitals, ideally, should have collaborated with local and national health authorities to combat such events. Unfortunately, that was not always possible because of wars, political unrest, or lack of capacity. Sometimes these hospitals did not have sufficient laboratory infrastructure to diagnose infections such as arboviral diseases. More emphasis should have been placed on enabling hospitals to both diagnose and control epidemics.
Key words: Mission hospitals, epidemics, Ethiopia
Although Ethiopia remains among the 20 poorest countries in the world and is the second most populous country in Africa, it has, in recent years, experienced improvements in living standards and health. Ethiopia includes 75% of all highlands above 2000 m in Africa and also contains the lowest points in the world. Thus, given these great topographical variations, the country includes multiple climate zones that cause large variations in ecology and vegetation, and thus, also in disease patterns.
In recent years, many researchers have published manuscripts describing ways to control infectious diseases. Although it is also theoretically possible to eradicate diseases, only smallpox has been eliminated. Recent statistics on malaria in Africa, along with the history of malaria eradication, reveal that although reducing the occurrence of the infection is possible, some even doubt if it is feasible to completely eradicate it.1 In the 1960s, the United States attempted to eradicate measles, but in recent years, it has returned with large numbers of cases both in the USA and in Europe.2 Hence, are there any roles for mission hospitals to contribute towards an outbreak response?
Since about 1950, the Norwegian Lutheran Mission (NLM) has been running several hospitals in Southern Ethiopia. Their work methods were to some extent influenced by the organization’s previous work in China, as well as the experience of British missionary doctors such as Dr. Stanley G. Brown, who practiced hospital work, research, and community engagement in order to alleviate disease burden.3 This work focused on practicing holistic medicine.4 The core priorities included, what have become, important elements in the development of health care in the UK and in Scandinavia: confidence, confidentiality, competence, contract, community responsibility, and commitment.5
The authors of this study worked in Ethiopia while smallpox was being eradicated and when Ebola was first described in the Congo. Based on earlier publications, we regarded Southern Ethiopia as a potential area for emerging infections, and we recorded unusual events. In addition, the epidemiology of commonly occurring infections had previously been poorly described. This manuscript presents a narrative concerning the mission’s response to previous epidemics, and the ways in which this was documented and operationalized in order to control the infectious disease outbreaks.
To our knowledge, many of the records from Southern Ethiopia are not publicly available. We, therefore, believe that this presentation is valuable for other mission hospitals and for future researchers attempting to learn about epidemics and potential epidemics and to intensify measures for meeting such challenges in the future.
The Norwegian Lutheran Mission began health care work in Southern Ethiopia in 1950.4 In general, these hospitals—located in Yirga Alem, Arba Minch, and Gidole—could perform basic and essential functions in surgery, gynecology and obstetrics, pediatrics, and internal medicine.6 Basic laboratory tests were available, including bacteriology, and in the 1980s we became aware of the highly prevalent resistance to commonly used antibiotics.7,8
Since these hospitals were among the few functional health institutions in Southern Ethiopia, patients from almost all of the Southern part of the country came for treatment. The main catchment areas, however, were the sub-provinces depicted as the colored areas in Figure 1. A study done in 1985 revealed that the primary catchment area of a given hospital comprised the population living within a 50-km radius of the institution.9 The population in the catchment areas was, in the 1980s, about 2.5 million people.

Figure 1. Map of Ethiopia showing the catchment areas of the hospitals included in this study
For this study, we utilized three information sources. The background information concerning the development of the health care system in Ethiopia is described by Schaller and Kuls, as well as by Berhane et al.10,11 When referring to previously published work on epidemics, we use the original publications as references. In addition, both of the authors have, from 1978 to 2020, been working at different hospitals and universities in Southern Ethiopia. During the course of our work, we registered outbreaks of epidemics in our notebooks. These records have not been published before, and we believe that such data could provide some historical information concerning the situation in the area. We also reviewed the archives of the hospitals, particularly from Gidole Hospital and Yirga Alem Hospital, to record the epidemics that may have occurred in the catchment areas of the hospitals.
The earliest countries affected by the HIV pandemic included Uganda and nations in Central and Southern Africa. The epidemic was first registered in Ethiopia in 1984. Since Yirga Alem hospital is located along the main transportation route from Kenya to Addis Ababa, and the HIV epidemic in Ethiopia originally spread via truck drivers along the main transportation roads, we feared that Yirga Alem Hospital would be affectedearly on by this disease.12 Therefore, in 1985, we performed an anonymized survey of all admitted patients to Yirga Alem Hospital. We obtained one of the earliest versions of the ELISA kit from Norway and analyzed the sera from 185 patients admitted to the hospital on one day. We did not have an ELISA reader since it was possible to observe color changes on the ELISA plates suggesting infection. At that time, only one patient had a slight color change, and we concluded that the prevalence of HIV infection was low among our patients. During the years 1986 to 1990, however, the prevalence of the disease increased dramatically, and a large proportion of the hospital admissions were for this disease.12
As soon as antiretroviral drugs were available and the first studies had been completed in Uganda in 1994,13 we initiated work to start treatment as we had not observed any reduction in the occurrence of the infection based on preventive measures carried out by local churches and communities. Unfortunately, neither the mission organization nor the Norwegian Agency for Development Cooperation (Norad) was willing to fund such treatments. This changed, however, when less expensive and generic antiretroviral drugs became available from India in 2002. Our HIV treatment work was based on experiences from Haiti, where they had initiated treatment without any advanced laboratory setup.19 We confirmed that it is possible to perform both testing as well as treatment without sophisticated laboratories. Our treatment protocol was based on HIV testing, clinical staging, and total lymphocyte counts. Our results were encouraging and similar to the good outcomes of early studies carried out in Europe; details about the studies are described elsewhere.14-16 As such, they became a model for the later nationwide efforts to scale up antiretroviral treatment in Ethiopia.
Before the 1930s, the epidemiology of malaria was not well known in Ethiopia. Italian researchers performed surveys between 1936 and 1941; however, that provided information on vectors and the prevalence of infection.10 In 1958, a severe malaria epidemic occurred in the Central Ethiopian provinces, with over 3.5 million cases and approximately 150,000 deaths, leading to the establishment of the Malaria Eradication Program.10 Unfortunately, due to a lack of sustainability and the emergence of resistance to the insecticide DDT, this program ended. The civil war between 1974 and 1991 altered Ethiopia’s political landscape and further complicated eradication efforts. Social and economic development halted, and the health system suffered.17 Thus, even though malaria was an ancient disease in Ethiopia, its epidemiology changed after the 1980s. In the autumn of 1980, a severe malaria epidemic was noted in Gidole. During October and November, about 80% of the hospital beds were occupied by severely ill malaria patients, often with multiple organ involvement and some with cerebral malaria. The patients received standard treatment with chloroquine in addition to supportive care. Diagnosis was based on microscopy of thin and thick blood slides. Most of the admissions, during this period, were patients coming from the lowlands. Both young children and adults were affected, suggesting that the population may not have obtained any prior immunity. The lowland villages were inhabited by people from the Gidole highlands who had been forcefully relocated to the malarious lowlands.18 This was the so-called “Villagization program” and was a part of the Communist regime’s efforts to politically control the population and settle them in villages, something similar to the collectivization that happened in the former Soviet Union.19 Thus, efforts to discuss this with the authorities were difficult for two reasons: people were not allowed to move back to their home areas, where there was a lower prevalence of malaria, and the government did not have resources to instigate preventive measures; indoor residual spraying was the tool used at that time. Concurrently, the local administration in Gidole was persecuting Christians, and the hospital had limited freedom of movement.
In 1992, health authorities at Shakiso south of Yirga Alem asked one of the authors (THH) to investigate a possible outbreak of yellow fever. Many patients had died, and many were icteric. Microscopy of their blood revealed heavy loads of the parasite Plasmodium falciparum. Since they had been treated with chloroquine, this was one of the first indications of chloroquine resistance in Southern Ethiopia, a phenomenon that was later confirmed by others.24 Following this event, we increasingly used quinine to treat Pl. falciparum infections.
Visceral leishmaniasis is a zoonotic infection that affects people living in areas where the disease occurs among a reservoir (animal, mainly rodents) population. During our work in Yirga Alem and Gidole, we recorded patients coming from the Segen-Woito, Genale, Dawa, and Galana lowland river basins.20,21 Many years later, these areas were confirmed to be endemic areas of this deadly infection, affecting a large proportion of the rural population.22 This demonstrates the importance of noting even rare events at outpatient departments as they could signify major public health problems farther away. Patients with this disorder came to the hospitals because they were gravely sick, were severely wasted, and had huge spleens. The diagnosis was done by spleen or lymph-node punctures through microscopic identification of intracellular parasites (amastigotes) in stained slides. Sometimes, we also cultured the parasite using the Novy-MacNeal-Nicolle (NNN) medium. By publishing such reports and actively seeking collaboration with national research institutions, control programs to improve the community diagnosis through rapid tests, such as the Direct agglutination test (DAT), and decentralized treatment, were established.23
Measles is a well-known disease in Ethiopia. Mothers know how to diagnose it, and the disease has many local names. It is probably the most important vaccine-preventable disease. The Expanded Program on Immunization (EPI) started in Ethiopia in 1980. By 2002, however, nationwide coverage had only reached 40%, and a survey taken in Southern Ethiopia in 2017 revealed that only 60% of children under the age of five were fully vaccinated.24
Before starting the EPI program at Gidole Hospital in 1980, we performed a serological survey of schoolchildren in the area for one week. Of 35 children aged 9–36 months examined for measles antibodies (ELISA method, Central Laboratory and Research Institute, Addis Ababa), 25 (71.4%) had low antibody values, and 6 (17.1%) had post-infection values. While conducting the survey, over 400 children with measles were admitted to the hospital and to Konso Clinic. These children were severely ill, and many were as old as 10 years of age, suggesting that they had not contracted the disease before. In a previous publication, we showed that a survey covering the preceding four months, carried out in 19 villages with 45,884 inhabitants and 14,452 children younger than 10 years of age, identified 1,536 deaths due to measles (a case fatality rate of 10.6%).25
We attempted to carry out a large-scale vaccination campaign covering two districts with about 200,000 people, but it partially failed because we started too late and also because the population refused to be vaccinated. The community beliefs or attitudes can be summarized as follows:
Outbreaks of typhoid fever and of bacterial dysentery (shigellosis) were often observed. The diagnosis was based on the clinical features, often in combination with laboratory tests such as the Widal test. During the 1980s, we had established a microbiological laboratory and analyzed stool cultures.
In addition, there are multiple historical records of cholera epidemics afflicting Ethiopia. In the Amharic language, the affliction is known as “November disease”, and routes of communication, markets, pilgrimages, and troop movements have always played a role in the spread of the disease. In 1971, 47 patients with “rice-water” diarrhea were observed at the Konso Clinic, affiliated with Gidole Hospital. Two of those patients died. This was a part of a much larger cholera outbreak that affected Southern Ethiopia, and the Norwegian Lutheran Mission, in collaboration with the Ministry of Health, vaccinated 28,722 people in the same area. Similar vaccination campaigns were carried out around the Yirga Alem area, where there was an even larger outbreak of cholera. This demonstrates the close collaboration between the public health authorities.
During the Communist period (1974–1991), there were several outbreaks of cholera. The largest occurred in the mid-1980s after the great famine that affected large parts of Southern Ethiopia.26 At that time, it was strictly forbidden to use the word cholera. The international organization Médecins Sans Frontières (MSF) published the occurrence of cholera and was expelled from the country.27 At the time, the government preferred the euphemism “Sudden Severe Dehydration Diarrheal Diseases” (S2D3). They actively supported the treatment of these patients by mission hospitals although it was strictly forbidden to announce the occurrence of the disease. In 1985, at Yirga Alem Hospital, approximately 320 patients were clinically diagnosed to have the disease over a period of six weeks, 9 (3%) of whom died. At the time, we had established a basic microbiological laboratory, and stool cultures from approximately 50 specimens revealed Vibrio cholera biotype eltor, the dominant strain in the seventh global cholera pandemic.28 This microbiological lab was a part of the routine work at the hospital, and we trained national staff to do the daily work. However, we were not able to use this laboratory on a large-scale because of scarcity of reagents and difficulties in importing them.
Relapsing fevers belong to a group of acute infections caused by arthropod-borne spirochetes. The louse-borne relapsing fever caused by Borrelia recurrentis is endemic in Ethiopia. Over the years, there have been many small outbreaks, especially among the prison populations, and the hospitals received permission to visit prisons in order to prevent such infections through de-lousing campaigns, improving access to washing facilities, and treating sick persons. Following the civil war, 1990–1991, however, a relapsing fever epidemic affected large parts of Ethiopia. The root cause was thought to be approximately 500,000 soldiers returning from the war in Eritrea to their homes in all parts of Ethiopia, thus, spreading the disease to many parts of the country.29
From 1987 to 1995, we ran two small health and demographic surveillance sites in Southern Ethiopia, from which we monitored demographic, nutritional, and infectious disease trends.30,31 Every second week, we visited approximately 500 households with 2,500 inhabitants in two areas (Arsi and Borana). In 1992, we observed a huge increase in the crude death rate, most of which was due to relapsing fevers (Figure 2; not previously published), although some was due to malaria. The high crude death rate in 1988 was caused by a malaria epidemic (Figure 2).
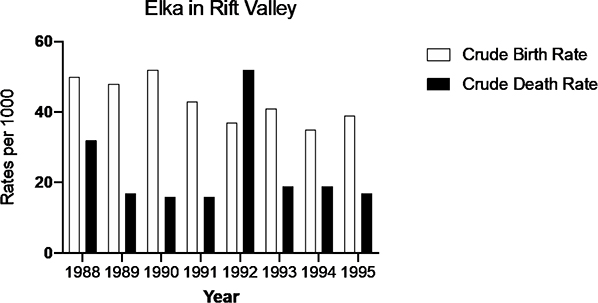
Figure 2. Crude birth and death rates in Elka (Arsi) showing an increase in the death rate during the relapsing fever epidemic in 1992
The relapsing fever outbreak of 1992 represents an unusual way for an endemic disease to become a national epidemic. Even if Southern Ethiopia had not experienced a major war, the unstable political situation, in combination with the lawlessness during that period and a weakening of the public health structure, meant that only those patients able to reach institutions such as mission hospitals received proper treatment.
Ethiopia was one of the last countries in which smallpox existed. The most severe epidemic was in the mid-1950s. Until it was eradicated in 1976, there were multiple outbreaks of smallpox.10 In the early 1950s, the government of Ethiopia made smallpox vaccination compulsory. The NLM-supported hospitals actively participated in vaccination campaigns and also in treating patients with smallpox. In the years after the last case of smallpox was observed, there was increased surveillance for possible smallpox cases in Southern Ethiopia. We reported several potential cases at Yirga Alem Hospital that were investigated by the Ministry of Health and, fortunately, not confirmed as smallpox.
Until 1980, the understanding was that peptic ulcer disease were rare in Africa, and, when Helicobacter pylori was established as its cause, it was declared that this did not concern Africa.32, 33 However, peptic ulcer disease was commonly seen at the hospitals, and many patients were operated upon for complications of this disorder. Therefore, and because of a collaboration with the University Hospital in Bergen in Norway, endoscopy of the upper gastrointestinal tract was introduced at the hospitals in the 1980s. Based on a simple and cheap staining of the mucus obtained during gastroscopy, it was possible to identify comma shaped Helicobacter pylori bacteria using methylene blue staining.34 Thus, within a few minutes, our patients received eradicative treatment for H. pylori nearly two decades before this infection was accepted as an etiological agent in Africa.35
In 1987, an unusual outbreak of acute conjunctivitis with profuse exudation was observed among 9,075 young children in Konso.36 Similar outbreaks of acute keratoconjunctivitis due to Neisseria gonorrhoeae were seen in the Nile delta in Egypt, the last time in 1948.37 Microscopy revealed gram-negative intracellular diplococci, and growth of penicillin-susceptible N. gonorrhoeae was confirmed at Arba Minch Hospital. Treatment was successful, using ampicillin plus probenecid as an oral single dose treatment. The epidemic stopped when the rains started in June 1988. On one occasion, Neisseria gonorrhoeae subspecies Kochii was identified, and our observation was the first known major outbreak in 40 years outside Egypt.37
The Northwestern section of Ethiopia is part of the meningitis belt of Africa. Before 1979, there had been no systematic studies on the distribution of meningococcal meningitis epidemics in other parts of the country.38 Although Southern Ethiopia lies outside the meningitis belt of the Sahel, records from mission hospitals show outbreaks of meningitis. In 1962, a meningitis epidemic was recorded in Gidole, with another occurring in 1979. Lumbar punctures revealed purulent spinal fluid, high cell counts, and low sugar. In 1981, additional cases were recorded from the Mashille villages south of Gidole, and gram staining of the patients’ spinal fluid showed intracellular gram-negative diplococci. In 1988, 1996, and 1997, numerous cases were reported from Konso and also at Yirga Alem Hospital. The largest outbreak observed at Yirga Alem coincided with the nationwide outbreak that occurred between 2000–2001.39
A large epidemic of yellow fever occurred in Southwest Ethiopia from 1960 to 1962.40 A Norwegian missionary doctor, responsible for the public health services in Gamo-Gofa Province during the same period, participated in the vaccination campaigns. Since then, there have been repeated outbreaks of yellow fever in the same area. In recent years, these epidemics were also investigated by Arba Minch University, which, through the support of former NLM missionary doctors, had established a molecular entomological laboratory.41 This laboratory was primarily established to do molecular studies of the malaria and leishmania parasites and the disease transmitting mosquitoes. When the yellow fever epidemic occurred, we also used the laboratory for such diagnosis. The laboratory is a part of Arba Minch University’s routine work and is run within a national and sustainable context.
We were well aware of reports of other hemorrhagic fevers that had occurred in Ethiopia. Dengue fevers had been reported by Italian researchers in 1940, and based on serological evidence, researchers from the Naval Medical Research Unit (NAMRU 3) had reported the occurrence of West Nile fever, Zika, Chikungunya, and Marburg-like viruses in Southern and Western Ethiopia.40,42 We clinically suspected Chikungunya infection in patients presenting with high fever, headache, and joint and muscle pain. This group of patients came from Bilate, just north of Lake Abaya, and at the hospital, the diagnosis of these patients was referred to as “Bilate disease.” Over the years, we attempted to obtain permission to take serum samples to the arbovirus reference laboratory in the United Kingdom but were not permitted to export the blood samples by the Ethiopian authorities. In retrospect, we should have established immunological tests earlier at our hospitals in order to confirm such outbreaks.
Over the years, the mission hospitals in Southern Ethiopia have observed multiple epidemics. Some of these events, such as shigella and typhoid fever epidemics, were expected, while at other times, the epidemics occurred in different forms and in unexpected scales. Given the large size of some of the outbreaks, it was often beyond the capacity of the mission hospitals to control these adverse events. The relapsing fever epidemic of 1991–1992 was such an occurrence. In hindsight, we should have invested more in laboratory efforts to diagnose such infectious diseases, especially in patients with unexplained fevers.
Several of the outbreaks we have described in this study have not been previously published. The reasons could be that the topic was too sensitive (e.g., cholera), while at other times, it was because there was limited time at a busy hospital to report and perform more in-depth investigations. The local ministries of health, however, were always informed about local outbreaks, since that was part of the standard routine.
Another limitation of our multiple case reports is that many of them were not population-based, although cases coming to the hospital often reflect what is going on in communities. David Morley, who worked as a pediatrician in Nigeria, stressed that physicians working at institutions should serve their communities.43 During busy work schedules at rural hospitals, this may be difficult. It remains the physician’s community responsibility, however, not only to treat patients, but also to understand the patient’s background and the environment in which they live.3 Often, community-based preventive interventions will save more lives than treating patients at hospitals.
At times, it was possible to collaborate with the national authorities to control disease outbreaks. At other times, this was beyond the capacity of the mission organization, and occasionally, the government opposed any involvement from a foreign organization. By maintaining a low profile and limiting strategies that could humiliate the government, however, it is usually possible to collaborate and develop practical solutions. Gill Walt has proposed that by focusing on small-scale politics, including micro-policies that are the responsibility of the local and regional Ministry of Health, it is usually possible to bring about policy changes.44
For every epidemic outbreak, early warning and early action are essential. However, mission hospitals have limited resources, and mission organizations usually do not budget for unforeseen events such as epidemics. It may be appropriate to suggest that mission organizations set aside some of their budget for such unforeseen events. Mission hospitals are well-placed to identify outbreaks. Furthermore, given their close collaboration with local, regional, and national governments, it is often possible to coordinate efforts, thereby limiting epidemic outbreaks.