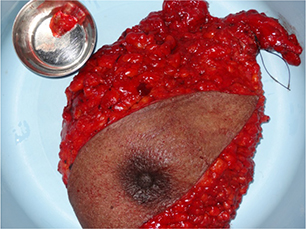
Figure 1. Left modified radical mastectomy specimen
CASE REPORTS
Royson Dsouzaa, Anish Jacobb, Mrudula Raoc, Nandakumar Menond
a MBBS, MS, Consultant Surgeon, ASHWINI - Gudalur Adivasi Hospital, The Nilgiris, Tamil Nadu, India
b MBBS, MS, MCH, Assistant Professor, Department of Endocrine Surgery, Christian Medical College, Vellore, India
c MBBS, MD, Medical Superintendent, ASHWINI - Gudalur Adivasi Hospital, The Nilgiris, Tamil Nadu, India
d MBBS, FACS, DABS, Director, ASHWINI Health System and Gudalur Adivasi Hospital, The Nilgiris, Tamil Nadu, India
The burden of breast cancer has been on the rise world over and has become the most common cancer among women in urban India and the second most common cancer in rural women after carcinoma cervix. There is a considerable delay in presentation, associated with a lack of access to adequate and timely surgical intervention. Consequently, most patients present to tertiary care centers in advanced or inoperable stages. Many subsets of these patients can be managed adequately in resource-limited rural surgical centers. In this series of patients diagnosed with carcinoma breast, we have outlined comprehensive management possible in resource-constrained settings. The challenges in adhering to the standard of care and strategies to overcome these limitations have been discussed with a relevant review of the literature.
Key Words: carcinoma of the breast, surgery, low resource, management, India
The Lancet oncology commission’s projections for new cancers by 2030 worldwide are estimated to be 21.6 million of which 17.3 million will require surgery. Of these, 10 million will be from Low-and Middle-Income Countries (LMICs) like India. In LMICs, three-quarters of the surgical burden will be from cancers of the breast, head and neck, esophagus, stomach, lung, cervix, and prostate.1 Breast cancer is the most common cancer among women in high income countries.2 With the rising incidence of breast carcinoma the world over, it has become the most common cancer among women in urban India and the second most common cancer among rural women after carcinoma of the cervix.2–5 The various myths as well as the prevalent ignorance common in Indian society are counterproductive in raising awareness about breast cancer.6 In addition, denial of the disease, fear of treatment, and social/financial issues contribute to the majority presenting late.4,5,7,8 This delay in presentation is especially true for patients coming from a lower socioeconomic status, and in addition to the above factors, there is a lack of access to adequate and timely surgical care for this subset of patients.4,9-11
The cases described in this series were managed at the Association for Health Welfare in the Nilgiris (ASHWINI), a health network that directly serves approximately 20,000 tribal population spread across the Nilgiris mountains of Gudalur and Pandalur in the state of Tamil Nadu, South India. There are five separate Adivasi tribes living in nearly 350 hamlets. ASHWINI healthcare is decentralized with eight area health centres that have two community health nurses who conduct outreach clinics in villages, screening for cancers among other healthcare activities. If patients are diagnosed with a suspicious breast lump during screening, they are then referred to ASHWINI Gudalur Adivasi Hospital for further management. The hospital has 50 well equipped beds with two operating rooms and facilities for ultrasound, plain radiography, laparoscopy, gastroscopy, and a laboratory and blood bank where the treatment is completely free. Through these series of cases, we describe various clinical presentations of breast cancer and our management strategies in each case.
Consent was obtained from each patient for their case to be reported.
A 64-year-old woman presented with complaints of a painless mass in the left breast for two months that rapidly progressed in size. There was no history of nipple discharge or skin changes. She attained menopause at 50 years of age and had normal menstrual cycles prior. There was no family history of BRCA-associated malignancies. On examination, there was a mass in the upper quadrant of the left breast measuring 5x4 cm that had an irregular surface, hard consistency, and restricted mobility. There was no infiltration into the underlying pectoral muscles or the skin. There was a single mobile lymph node palpable in the left axilla. The right breast and axilla were unremarkable.
With a clinical suspicion of carcinoma left breast, the patient underwent further evaluation. Breast ultrasonogram (USG) showed an irregular hypoechoic mass in the left breast without axillary lymphadenopathy characterized as a BIRADS 5 lesion. A core biopsy confirmed invasive carcinoma of the left breast. Following this, the patient underwent upfront surgery. In view of the palpable axillary lymph node, a left modified radical mastectomy (MRM) was performed (Figure 1). The final histopathology was consistent with invasive carcinoma of the left breast T2N0, immunohistochemistry (IHC) showing ER and PR positivity while Her2 neu was negative. The Ki67 index was reported to be 7%. The patient received hormonal therapy alone with tamoxifen and was well on annual follow up.

Figure 1. Left modified radical mastectomy specimen
A 55-year-old woman presented with a history of a painless mass in the right breast that progressively increased in size over 2 months. There was no involvement of skin or nipple areola complex. She underwent a hysterectomy at 40 years of age for fibroid uterus. There was no family history of BRCA-associated malignancies. On examination, there was a mass in the upper quadrant of the right breast measuring 3x3 cm that was firm, irregular, and with restricted mobility. The right axillary lymph nodes were not palpable. The left breast and axilla were normal.
An USG of the right breast showed a BIRADS 5 lesion without axillary lymph-adenopathy. A core biopsy was suggestive of mucinous carcinoma which was positive for ER, PR, and negative for Her2neu. As she had an early breast cancer with favorable IHC, she was offered upfront surgery. Due to the COVID-19 pandemic and travel restrictions, mammography was not obtained, as the closest facility for the same was three hours away. The patient requested breast conservation surgery (BCS) and was motivated to undergo post-operative radiation therapy as well as frequent follow up. BCS was performed along with level 1 and 2 right axillary lymphadenectomy (Figure 2). The histopathology confirmed mucinous carcinoma with adequate margins and absent lymph nodal metastasis (T2N0, ER+, PR+, Her2 neu -, Ki67 index 7%). As a radiation therapy facility was not available at our hospital, she completed 25 cycles of radiation therapy at a cancer center and is on regular follow up on hormonal therapy without evidence of disease recurrence after 1 year.
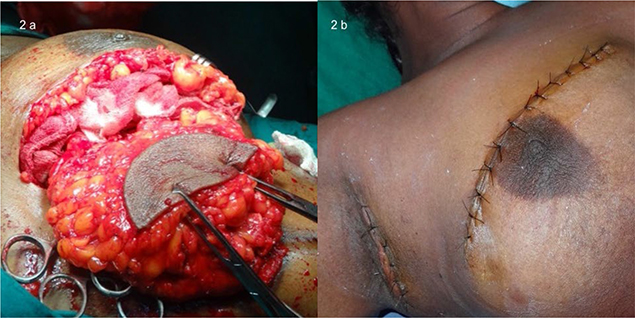
Figure 2. Breast conservation surgery with level 1 and 2 axillary lymphadenectomy
A 45-year-old woman presented with complaints of a painful mass in the right breast for 3 months. On examination, she was severely emaciated and had features of systemic inflammatory response syndrome (SIRS) with hypotension. There was an 8x6 cm ulcero-proliferative growth occupying the entire right breast destroying the nipple areola complex. The growth had copious foul-smelling discharge and maggots. It was tender on palpation with superficial infection and was fixed to the chest wall. There were multiple right axillary lymph nodes palpable. Her haemoglobin was 7.6g/dL, ALP was 391U, and chest radiograph showed bilateral pleural effusion.
The nature of disease and prognosis was explained to the patient’s family. In view of advanced disease and poor outcomes, they opted for non-escalation of treatment. The patient was offered best comfort care with local debridement and palliative chemotherapy and succumbed to the disease within a week.
A 47-year-old woman with type 2 diabetes mellitus presented with a slowly progressive left breast lump for 6 months. There was no history of skin changes or nipple discharge. On examination, there was a 2x2 cm firm lump with restricted mobility in the upper outer quadrant of the left breast. It was free from the underlying muscle and overlying skin. The left axillary nodes were not palpable.
Breast USG showed a solid, irregular lesion without axillary lymphadenopathy (Figure 3). Core biopsy suggested a non-specific chronic inflammatory lesion without evidence of malignancy. A lumpectomy was done but the final histopathology was consistent with invasive carcinoma. She then underwent a modified radical mastectomy, the histopathology reporting invasive ductal carcinoma T2N0 which was triple negative (TNBC) with Ki67 index of 67%. She underwent adjuvant chemotherapy at a tertiary center and was well on follow up.
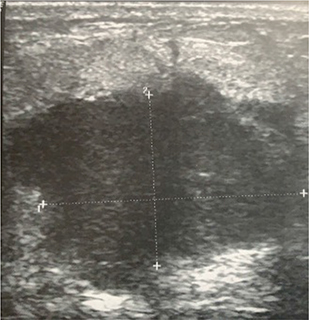
Figure 3. Ultrasonography image of the left breast showing a solid irregular lump
The management of breast cancer has been rapidly evolving with an increased focus on multimodal therapy which includes surgery, radiation therapy, chemotherapy, targeted therapy, and hormonal therapy. Working as general surgeons at a tribal secondary care hospital in India, with limited diagnostic facilities and non-availability of an oncologist and a radiation therapist, we have made an attempt to describe how best such patients can be managed.
Currently, breast cancer awareness programs in India are focused to cities and have not reached the rural areas.5,6 A rural surgeon caters to a defined population and is ideally suited to spearhead screening programs for the detection of common cancers in that geographic location.12 In addition, through these outreach programs, health education regarding breast cancer with special emphasis on risk factors, symptoms, and breast self-examination can be taught.3,4 ASHWINI integrated healthcare has dedicated community health nurses, village health volunteers, and animators who conduct outreach clinics where health education and screening are performed. They encourage patients to seek appropriate care when breast lumps are detected. The rapport they have with the local community help them educate the community with regards to the various myths as well as the prevalent ignorance that is common, building an awareness about breast cancer. In addition, they can help alleviate fear of the disease and its treatment as well as aid access to adequate and timely surgical care for this subset of patients. This has resulted in early detection of breast cancer in patients and treatment with curative intent. This may be a model that can be replicated in other rural areas for breast cancer screening and education.
The importance of clinical evaluation cannot be over emphasized in the diagnosis of carcinoma breast.13 This is even more important in rural areas where the options for second opinion and aid of advanced imaging are scarce. Triple evaluation, that comprises clinical history and examination, imaging with ultrasound and mammography, and pathological tests with core biopsy have increased the overall diagnostic accuracy in detecting breast malignancies.14 Mammography is not available at our hospital, necessitating reliance on ultrasonography for breast imaging. Similarly, due to the lack of an in-house pathologist, core biopsy specimens need to be transported to the closest tertiary care center (5 hours away) for reporting, and the results are available only after 10–14 days. These limitations cause a delay in the initiation of appropriate treatment.
Ultrasonography (USG) is a valuable addition to a general surgeon’s diagnostic acumen and is rapidly becoming an integral part of clinical practice.15,16 Non-availability of a radiologist at our centre makes it crucial for the surgeon and family physician to perform USG. A rural surgeon should be competent to perform diagnostic ultrasound of the breast, thyroid, focused assessment with sonography in trauma (FAST), and vascular systems.15 USG can often resolve the clinical dilemma of various breast lumps.17 Moreover, it is a helpful tool in performing focused aspiration cytology or core biopsy. Similarly, USG of the abdomen is the diagnostic modality of choice for evaluation of intraperitoneal metastasis. In Case 2, BCS was performed after ruling out multifocal disease solely on USG. Although mammography is crucial before planning a BCS, if circumstances do not allow for the same, a good USG evaluation can still be used.18
FNAC is an inexpensive, easily available, and easy to perform procedure that has a good sensitivity and specificity for detection of breast cancer. FNAC has been the diagnostic procedure of choice for patients with breast lumps in the past. Core biopsy increases diagnostic accuracy in addition to providing tissue for immunohistochemistry that, in turn, helps in prognosticating the disease.19 Currently, the primary modality of treatment for patients with breast cancer is dependent on hormone receptor status. Although these tests can be easily performed, the availability of a pathologist and procurement of core biopsy needles are a challenge in rural centers. As mentioned earlier, we do not have an inhouse pathologist and have to rely on the closest tertiary care center. Core biopsy needles are procured as donations from tertiary care centers, which are re-used after adequate sterilization to minimize costs. In spite of the above hurdles, we perform core biopsies on all breast lumps as this is the standard of care the world over.
The management of breast cancer is rapidly evolving. Therefore, multidisciplinary, team-based meetings for appropriate management of patients with carcinoma of the breast have taken a big lead in recent years.20,21 This team consists of endocrine and breast oncosurgeons, radiologists, pathologists, medical and radiation oncologists, and breast care nurses.22 This is not possible or practical in rural hospitals where the surgeon must play a paramount role in diagnosis, staging, and planning treatment. However, with the advancement in technology and connectivity, online MDT meetings can be scheduled in collaboration with tertiary care centers that can help in planning optimal management for each patient.23 In our series, Cases 2 and 4 were discussed in an MDT meeting with a tertiary care centre via emails and online meeting platforms and appropriate decisions were made.
MRM remains the most common procedure performed for breast cancer in India.10 With the evolution of chemotherapeutic agents, modalities for radiation, and hormone therapy, the extent of surgery has reduced considerably.22,24 However, adherence to adjuvant therapy remains questionable among patients from rural areas.4,8 This lack of adherence is a problem we face at our hospital as well. In addition, lack of a radiologist and reliance on USG of the breast performed by a surgeon for the assessment of the axillary nodal status has resulted in MRM being the most common surgery performed at our hospital for breast cancer.
BCS in rural surgical centers is neither popular among our patients nor our surgeons. There is a significant disparity, with BCS being more often received by patients in urban areas, higher socioeconomic status, and in areas with better accessibility to cancer care.25 Though multiple studies have showed similar oncological safety between BCS and MRM, the need for post BCS radiation to the breast limits its acceptance in rural hospitals.26-27 As seen from our series, there is a small subset of patients motivated for BCS and willing to undergo adjuvant radiation even if they have to travel to another centre for the same. A lot depends upon the rapport between the surgeon, the patient, and the family and the counselling conducted. Therefore, we feel that the onus is on the surgeon to identify and offer conservation to those who are motivated.
In patients with early breast cancer and node negative axilla on clinical/imaging, sentinel lymph node biopsy (SLNB) has emerged as the standard of care for the surgical assessment of the axilla. This may be performed either by a dual tracer technique or a single dye technique. Methylene blue dye alone has been showed to be safe, simple, and cost-effective with acceptable identification and positivity rates. Therefore, in this subset of patients, SLNB with methylene blue dye alone should be offered for the assessment of axillary lymph node status.7,24,28,29 Though we have not performed SLNB in the cases presented, this is a future direction we hope to take.
Mastectomy influences the patient’s emotional stability leading to fear, hopelessness, depression, and a negative attitude about their body.2 Breast reconstruction is an integral part of management of carcinoma of the breast, as it restores positive body image and quality of life in the patients.9 Although the emphasis on reconstruction is shown mostly by breast focussed surgeons, rural surgeons can very well adapt it.30 Options like microvascular free flaps may not be possible in rural hospitals due to the lack of infrastructure. However, pedicled flaps like pectoralis major and latissimus dorsi myocutaneous flaps are still feasible. Moreover, secondary care hospitals are not limited by time constraints and long waiting lists that are common problems in tertiary care centres.4
Systemic therapy has become the cornerstone in the management of breast cancer. The development of newer chemotherapeutic agents has resulted in improved prognosis and survival. In addition, targeted therapy has positively impacted the management of patients with Her 2 positive breast cancers. Though most secondary care centers lack the expertise to provide systemic/targeted treatment, all these modalities can be made available and administered in a rural setting with help from medical oncologists at other centres.31 Closer co-operation must be fostered between medical oncologists and surgeons to tailor simpler, cheaper, and less toxic chemotherapy regimens for patients who, for various reasons, are unable to go to tertiary care centers. Hormonal therapy that is relatively inexpensive can be offered with greater interest as it is easier to administer and likely to have better compliance. Radiation therapy on the other hand requires dedicated infrastructure as well as a trained radiation therapist. One must be able to counsel patients who require radiation to attend therapy at available centers.
In our experience, often patients have declined adjuvant treatment following surgery or have not completed adjuvant therapy due to financial constraints. Our patients face two major types of cost: firstly, the direct cost associated with adjuvant therapy, admission, investigations, and the non-medical costs like transport, food, and lodging. Secondly, the indirect costs due to loss of productivity and labor losses of the patient and family are significant. These areas are where government or non-government organizations will need to lend financial support. There are multiple opportunities for financial aid available for cancer patients, and the primary care provider and the rural surgeon—should be aware of the same. They can play a major role in mobilizing financial aid for these patients.
As depicted in Case 3, a rural surgeon should be equipped to manage patients with locally advanced/metastatic disease who present in poor general condition. These patients require best comfort care with psychological and social support for the family.
Providing standard care at rural surgical centers is very challenging due to the lack of resources and expertise. However, as depicted in our series and discussion, a rural surgeon can overcome these limitations and provide optimal treatment for patients with breast cancer. In addition, the presence of community health care nurses and village volunteers can aid in the early detection of breast cancer.