Figure 1. Flow Chart of Recruitment
ORIGINAL ARTICLE
Litha Mary Mathewa, Leejia Mathew b, Verghese Cherianc, Alice Davidd
a MBBS, DA, DNB, Consultant, Anesthesia Department, Believers Church Medical College, Kerala, India
b MBBS, MD, Dip Pal Med, Consultant, Anesthesia Department, Believers Church Medical College, Kerala, India
c MBBS, MD, FFARCSI, Professor of Anesthesiology, Penn State Health College of Medicine, USA
d MSc., PhD, Head of Medical Research, Epidemiology & Biostatistics, Believers Church Medical College, Kerala, India
Background and Aims: Managing the pain after a Total Knee Arthroplasty (TKA) is essential for early mobilization and rehabilitation, which plays a crucial role for better clinical outcomes. Epidural infusion of local anesthetic and opioids provides good pain relief but can lead to side effects such as hypotension, motor weakness and respiratory depression. The objective of this study was to evaluate if epidurally administered ketamine could provide postoperative analgesia, thereby, reducing the dose of epidural infusion and the need for rescue analgesia.
Methods: Thirty patients undergoing TKA under epidural anesthesia were randomized to receive 0.5% bupivacaine (Group I) or 0.5% bupivacaine + ketamine (0.5mg/kg) (Group II) as their primary anesthetic. At the end of the surgery, an infusion of 0.1% bupivacaine + fentanyl (1μg/ml) was started through the epidural catheter at 5ml/h. The rate was adjusted every 2 hours, depending on the pain experienced by the patient. If, despite rate adjustment, the patient graded the pain as 5 or more, morphine 5mg intramuscularly could be administered as the rescue analgesic.
Results: The dose of epidural infusion in the postoperative period between the 2 groups was comparable. Rescue analgesia was needed in 5 (35%) Group I and 8 (53%) Group II patients. An analysis of the subset of patients using the Kaplan-Meier curves, showed that most of the patients from Group I needed the rescue dose at the sixth hour. Few of the Group II patients also needed rescue analgesia at the sixth hour but their rate of needing rescue analgesic was gradual, lasting up to 18 hours.
Conclusion: This study showed that the addition of a single dose of ketamine (0.5mg/kg) did not improve postoperative analgesia after TKA. However, it may have some benefit in a select subset of patients.
Key words: epidural, ketamine, pre-emptive analgesia, total knee arthroplasty, post-operative pain
Total Knee Arthroplasty (TKA) is often followed by pain, requiring analgesics in the post- operative period to facilitate early mobilization and rehabilitation.1 One of the techniques of providing post-operative analgesia in these patients is the administration of a local anesthetic through an indwelling epidural catheter. However, this technique could be associated with hypotension, blockade of motor function, and tachyphylaxis.2 Various additives such as opioids, alpha 2 agonists,3 midazolam,4 and ketamine5 have been shown to reduce the dose of bupivacaine thereby retaining effective analgesia with reduced dose-related side effects.6
Identification of opiate receptors in the spinal cord has led to widespread use of intra spinal opioids for postoperative pain management;7 however, this is associated with side effects such as nausea, vomiting, pruritus, urinary retention, and delayed respiratory depression.8
The understanding of the role of N-Methyl-D-Aspartate (NMDA) receptors in pain modulation has prompted the use of NMDA receptor antagonists such as magnesium and ketamine to control postoperative pain.9 Ketamine, administered intrathecally or epidurally, targets the NMDA receptors located within the dorsal horn10 and has been found to be effective in controlling postoperative pain.11 This study was undertaken to assess if addition of ketamine to epidurally administered bupivacaine, improves postoperative analgesia after TKA surgery.
This randomized, double-blind, placebo-controlled study was approved by the Institutional Research and Ethics Committee (IRB Min No. 5491). All ASA (American Society of Anesthesiologists) physical status 1 or 2 patients scheduled for elective TKA were eligible to be enrolled in this study. Patients with spinal abnormalities, pre-existing neuropsychiatric illness, chronic pain syndromes, or those with any contraindication to epidural anesthesia or use of ketamine were excluded from participation. The study was explained to all eligible subjects and a written consent was obtained from all those who volunteered.
In the operating room, after establishing standard monitors and administering intravenous fluids, an epidural catheter was placed by the anesthesiologist on record, who was blinded to the randomization. The participants were randomized to one of two groups. Group I received 10ml of 0.5% bupivacaine and Group II received 10ml of 0.5% bupivacaine + ketamine (0.5 mg/kg body weight). The anesthesiologist was permitted to administer aliquots of 0.5% bupivacaine to achieve sensory block for surgical anesthesia. The patient’s vital parameters were monitored every 5 min for the duration of the surgery. At the end of the surgery, an infusion of 0.1% bupivacaine + fentanyl (1μg/ml) was started through the epidural catheter at 5ml/h. The rate was adjusted every 2 hours, depending on the pain experienced by the patient as assessed by a 11-point Numerical Rating Scale (0–10). If despite rate adjustment, the patient graded the pain as 5 or more, morphine 5 mg, intramuscularly, was administered as the rescue analgesic. All patients were monitored for 48 hours after surgery for pain relief, sedation, incidence of vivid dreams or hallucinations, and sleep disturbances. Sedation level was measured as 0 – awake, 1 – responds to verbal call, 2 – responds to tactile stimulus, 3 –unresponsive. Patient satisfaction was also measured using a 6-point NRS (0–5) at 48 hours. The epidural administration of bupivacaine and fentanyl was continued for 48 hours.
Prior to the start of the study, a computer- generated random list, in blocks of 10, was generated and serially numbered sealed envelopes containing the group number were created. The envelope was opened after the epidural was sited and an anesthesiologist, not involved in the study, was asked to load the study drug and hand it over to the anesthesiologist who sited the epidural.
The means of each group were compared using Student’s t-test. The proportions were compared using Chi square test and the Fisher’s exact test was used whenever the expected cell values were less than five. A p-value less than 0.05 was considered to be statistically significant in all the above tests. Time to the first rescue analgesia was compared using the Log rank test and graphically represented using Kaplan Meier Curves.
Thirty patients scheduled for TKA were randomized to either Group I (bupivacaine) or Group II (ketamine + bupivacaine). One patient in Group I had to be withdrawn because of failure to achieve adequate surgical anesthesia with epidural and had to be administered general anesthesia. The flow chart according to the Consort guidelines for RCT is given in Figure 1. The baseline characteristics of patients in the two groups were comparable. See Table 1. Postoperative pain was well controlled in all the study patients. The dose of bupivacaine and fentanyl administered epidurally was comparable between the two groups. See Table 2. Despite adjusting the infusion rate of the epidural solution, eight subjects in the ketamine group and five in the control group needed additional analgesic in the form of morphine (5mg) intramuscularly, but this was not statistically significant. Among those who needed it, the average time to rescue analgesia was on an average 3h later in the ketamine group. See Table 3. A further analysis of the subset of patients who needed rescue analgesia was done using the Kaplan-Meier curves. Most of these patients from Group I needed the rescue dose at the sixth hour. Although few of the Group II patients also needed rescue analgesia at the sixth hour, their rate of needing rescue analgesic was gradual, lasting up to 18 hours. See Figure 2. This difference was not statistically significant as measured by the Log-rank test (p=0.1564).
The blood pressure, the heart rate and the oxygen saturation were stable and comparable between the two groups. During the postoperative period the incidence of complications such as nausea and vomiting, urinary retention and paresthesia was comparable between the two groups as was the level of sedation (p >0.05). None of the patients complained of hallucinations or vivid dreams. Patient satisfaction at 48 hours was not statistically different. See Table 4.

Figure 1. Flow Chart of Recruitment
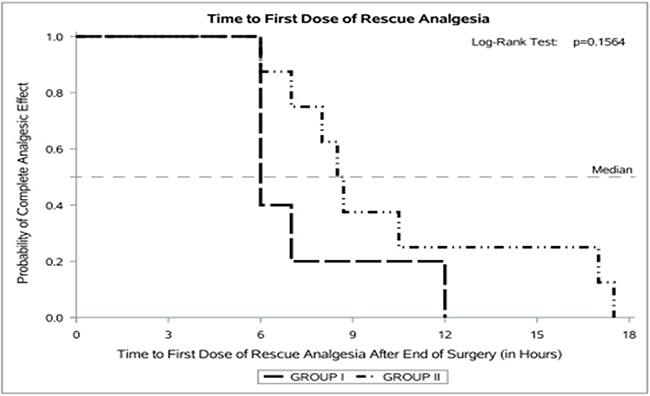
Figure 2. Kaplan Meier Curve Comparing Those who Received Rescue Analgesia in Group I (bupivacaine) and Group II (bupivacaine + ketamine)
The surgical technique and the postoperative management of TKA have evolved since its inception over 50 years ago. Epidural infusion of local anesthetic with adjuvant analgesic medication used to be an accepted mode of analgesia for this procedure. Due to the associated motor weakness, attempts were made to reduce the concentration of the local anesthetic, while changing the adjuvants to minimize side effects. In developed countries, pain management strategy has moved from epidural infusion of local anesthetic to continuous selective nerve block with or without infiltration of the local tissue with a solution containing a mixture of ropivacaine, epinephrine, ketorolac, and saline which is infiltrated around the knee joint by the surgeon after skin closure. Transition to minimally invasive surgical technique which causes limited tissue injury and associated pain may have contributed to this change in pain management. Moreover, the offer of a “bundle package” by the insurance companies incentivized reducing the hospital stay. With improved home nursing care, it was cheaper for the insurance company to manage pain at home using infusion pumps to deliver local anesthetics into catheters placed in proximity to peripheral nerves.
However, in developing countries, where community-based healthcare facility is nascent, and poor hygiene and low socio-economic conditions preclude the use of sophisticated infusion pumps in a home setting, techniques such as epidural analgesia and care in a hospital ward for a couple of days are still very common, practical, and affordable.
A continuous infusion of local anesthetic through an epidural catheter is a well-established technique of providing postoperative analgesia. However, to reduce the motor blockade, the concentration of local anesthetic is kept low and opioids are added to complement the analgesic action. The addition of opioids can cause side effects such as pruritus, sedation and respiratory depression.8 In many developing countries, the limited availability of opioid medications, the paucity of monitoring devices and inadequate staffing have prompted efforts to reduce the dose of opioids in the postoperative period and add a non-opioid based regimen for postoperative analgesia.12 Ketamine, readily available even in smaller towns, in low doses has been shown to be an effective analgesic with negligible hallucinogenic or respiratory depression effects.9
This study was an attempt to see if addition of a low dose of ketamine given epidurally, along with the initial dose of local anesthetic as part of the anesthetic for TKA, would reduce the dose of epidural infusion over 48 hours needed to control pain after the surgery. As explained above, a 48-hour infusion is still in practice in resource poor settings.
The study showed that addition of ketamine (0.5mg/kg) to bupivacaine given epidurally, did not reduce the requirement of the infusion of 0.1% bupivacaine + fentanyl (1μg/ml), administered epidurally, for postoperative analgesia. This is consistent with the findings of the study done by Weir et al.13 They added 3 different doses of ketamine (0.3 mg/kg, 0.5 mg/kg & 0.67 mg/kg) to 0.5% bupivacaine for epidural anesthesia in patients undergoing TKA and concluded that a single bolus injection of ketamine had no effect on the quality or duration of epidural analgesia. They concluded that since drug binding to the NMDA receptor occurs slowly, a bolus dose of ketamine may not provide adequate exposure of the drug to the receptor for maximal effect and speculated that a continuous infusion may prove more effective. Similarly, Yanli et al., in a placebo-controlled clinical trial added 25 mg ketamine to 20 ml of 0.5% bupivacaine administered epidurally to patients undergoing lower abdominal or orthopedic surgery. There was a small but significant decrease in onset time to anesthesia and a slightly higher segmental blockade in the ketamine group, but there was no difference regarding postoperative analgesic requirements.14 In contrast to the above studies, Himmelseher et al. used a mixture of S(+) ketamine and ropivacaine in a patient-controlled epidural analgesia (PCEA) system and showed better control of postoperative pain.5 S(+) ketamine has a four times higher affinity for NMDA receptors and twice the analgesic potency compared to racemic ketamine. In our study, due to the non-availability of S (+) ketamine, racemic ketamine was used. The PCEA system was not used either.
Rescue morphine was given intramuscularly since it was considered to be safer than intravenous in a ward with minimal nursing personnel and monitoring facilities. The analysis of those patients who needed rescue analgesia suggests a slight prolongation in analgesia among those who received ketamine (Figure 2). Therefore, a larger initial dose or an infusion of ketamine in the postoperative period may benefit even this subset. However, this study was not powered enough to delineate the characteristics of such patients.
NMDA receptors maintain neuroplasticity and hyperalgesia after a painful stimulus, as shown by both electrophysiological and behavioral studies carried out in animals.13-15 Hence, blockade of NMDA receptors preemptively might inhibit central sensitization to pain induced by tissue trauma and inflammation. Ketamine, an NMDA receptor blocker, has been shown to be effective in opioid dependent patients as well as those with chronic pain.9
The concept of preemptive analgesia suggests that the administration of an analgesic or local anesthetics before incision, might reduce the barrage of nociceptive stimulus induced by surgical trauma and, thus, the intensity of postoperative pain.16,17 Ketamine has been shown to provide better postoperative analgesia after laparoscopic hysterectomy when administered before the surgical incision.18,19 Ozyalcin et al., in a randomized, double blinded and placebo-controlled study, demonstrated that ketamine given epidurally compared to intramuscular was more effective in reducing perioperative analgesic requirements, hyperalgesia and touch allodynia after thoracotomy.20 Ketamine has been shown to improve postoperative analgesia in patients undergoing spine surgery and who have chronic pain and are opioid tolerant.21-22
Other modalities of pain control after TKA include administering local anesthetic through a peripheral nerve catheter placed in the adductor canal or infiltrating it into the periarticular tissue. A systematic review and meta-analysis of randomized controlled trials to evaluate the efficacy and safety of local infiltration anesthesia versus epidural analgesia for postoperative pain control in TKA23, has demonstrated that local anesthesia provides an equivalent efficacy in pain control with an increase in the range of motion and a reduction of the occurrence of nausea and length of hospital stay. However, the advantage of epidural is that it can be used as the primary anesthetic as well.
This study did not show any increase in the incidence of any side effects attributed to ketamine, such as increased sedation, hallucinations or vivid dreams. Nor was there any increase in complications such as nausea, vomiting, urinary retention, or paresthesia. Similarly, there was no difference in the level of satisfaction with the perioperative care between the two groups.
Although this study failed to show that the addition of a single dose of ketamine (0.5mg/kg) improved postoperative analgesia after TKA, it may have some benefit in a select subset of patients. A larger sample size would be needed to identify those patients. Perhaps the use of S-ketamine or addition of ketamine in the epidural infusion solution during the postoperative period may improve postoperative analgesia after TKA.